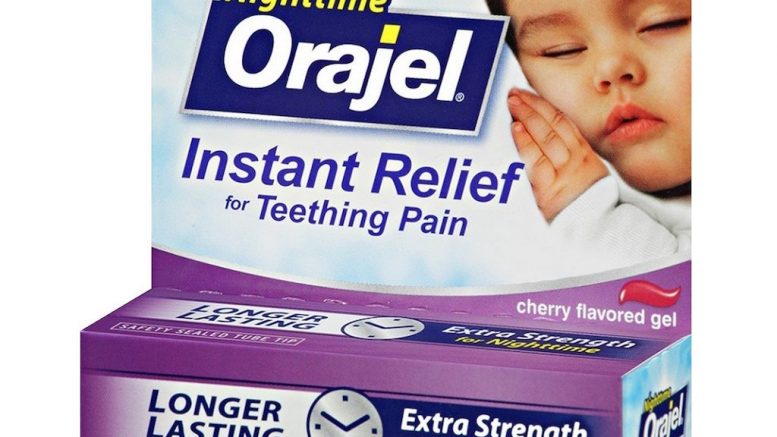
Teething remedies are popular for many parents looking to soothe their children’s teething pain. Often, teething medications like the popular Orajel contain a numbing agent called benzocaine.
The FDA claims that benzocaine can cause rare side effects, particularly in those under the age of two.
The agency has been issuing warnings for the past ten years, however, now they feel that due to no improvements, these medications should no longer be legally sold.
“We urge parents, caregivers and retailers who sell them to heed our warnings and not use over-the-counter products containing benzocaine for teething pain,” said FDA Commissioner Scott Gottlieb in a statement.
The FDA is also threatening the measure of legal action for companies that don’t remove the product.
Benzocaine is also used by adults for things such as canker sores and tooth aches. The FDA wants more extensive warnings on adult products, but hasn’t moved to have them banned.

Be the first to comment on "FDA Warns Teething Medicines Are Unsafe For Babies and Toddlers"